Accelerate Antibody-Based Therapeutics
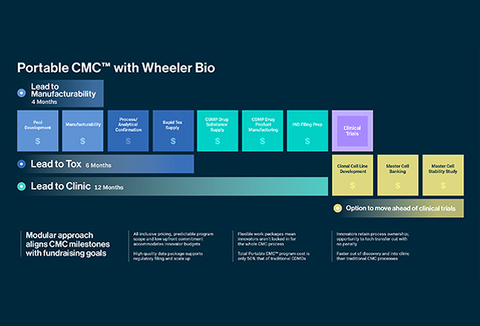
Wheeler Bio is a biologics CDMO that leverages the ModularCMC® approach, the cornerstone of its commitment to streamlining transitions from discovery to clinical-scale manufacturing. The ModuclarCMC® approach packages custom drug development and CMC services into modules that allow developers to mitigate cost risks and control timelines through defined scopes of work and specified deliverables.
Wheeler is proud to currently offer best-in-class mammalian cell line and process development, GLP Tox material supply, and efficient cGMP clinical supply. Connect with our team today to learn more about why Wheeler is the antibody CDMO partner of choice.
A Biomanufacturing Ecosystem

Oklahoma City, OK

Oklahoma City, OK
-
Stable Cell Line Development
-
Preclinical Material Supply & Testing from Stable Pools
-
Process Development & Optimization
-
Process Characterization
-
Analytical Development & Qualification
-
Formulation Development
-
Preclinical Material Supply & Testing from Stable Clones
-
cGMP Master Cell Banking
-
Grade C Solution Preparation
-
Grade D Ballroom Clinical Production Suites
-
QC Testing & Release
-
cGMP Drug Substance Manufacturing
News + Events
Proven Successes
Push Boundaries. Effect Change.
We are a growing team of CMC experts who share a passion for speed to clinical impact through collaboration.