Wheeler Bio Speeds Up and Simplifies the Discovery-to-IND Process.
Wheeler Bio brings biologics drug innovators, discovery CROs, and CDMOs together to solve an outdated industry bottleneck with a unique partnering model, built on shared success and lasting collaborations. The typical momentum drop between discovery and development poses significant challenges for emerging biopharma companies adding technical, financial, and regulatory risks. We believe these lifecycle challenges can be reduced with more democratization of manufacturing platforms.
Wheeler is proud to currently offer best-in-class mammalian cell line and process development, GLP Tox material supply, and efficient CGMP clinical supply. Connect with our team today to learn more about how Wheeler can add value to your project.

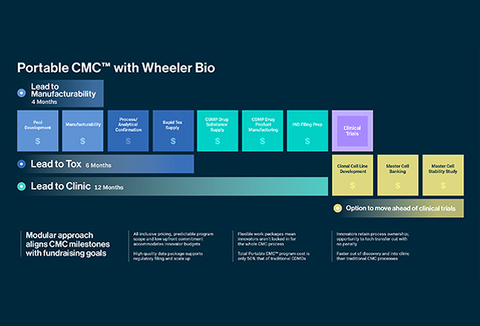
Get to IND Faster with Portable CMC®
With Portable CMC®, Wheeler is simplifying the connection between drug discovery and clinical supply by standardizing and democratizing the pathway from innovation to first-in-human clinical trials. A new bridge for translating discoveries to IND filing, innovators benefit from increased momentum during technology transfer, reduced risk, and modular work packages designed to fit timelines and budgets with ease.
Learn MoreA Biomanufacturing Ecosystem

Boston, MA

Oklahoma City, OK

Oklahoma City, OK
-
Molecular Biology
-
Transient Protein Production in HEK or CHO Cells
-
Preclinical Material Supply & Testing from Stable CHO Pools
-
Advanced Cell Line Engineering
-
Transient Protein Production in HEK or CHO Cells
-
Stable Cell Line Development
-
Preclinical Material Supply & Testing from Stable Pools
-
Process Development & Optimization
-
Process Characterization
-
Analytical Development & Qualification
-
Formulation Development
-
Preclinical Material Supply & Testing from Stable Clones
-
CGMP Master Cell Banking
-
Grade C Solution Preparation
-
Grade D Ballroom Clinical Production Suites (50L and 500L Scale)
-
QC Testing & Release Powered by Charles River RightSource℠ Technology
-
CGMP Drug Substance Manufacturing
News + Events
Proven Successes
Push Boundaries. Affect Change.
We are a growing team of drug development professionals who share a passion for speed to clinical impact through collaboration.